Telomeres may be the key to better understanding cancer and aging
/For years, we’ve thought of telomeres as markers of aging because they shorten as creatures grow older.
But telomeres, structures made from DNA sequences and proteins found at the ends of chromosomes, may serve as a kind of Rosetta stone that may help us better understand cancer and aging.
Researchers at the University of North Carolina School of medicine have discovered that telomeres contain genetic information to produce two proteins, one that is elevated in some human cancer cells, as well as cells from patients suffering from telomere-related defects.
Their study, published in the “Proceedings of the National Academy of Science,” found that telomeres do more than serve as chromosomal end caps that simply prevent chromosomes from fusing to one another.
"Based on our research, we think simple blood tests for these proteins could provide a valuable screen for certain cancers and other human diseases," said study co-author Jack Griffith, the Kenan Distinguished Professor of Microbiology and Immunology and member of the UNC Lineberger Comprehensive Cancer Center. "These tests also could provide a measure of 'telomere health,' because we know telomeres shorten with age.”
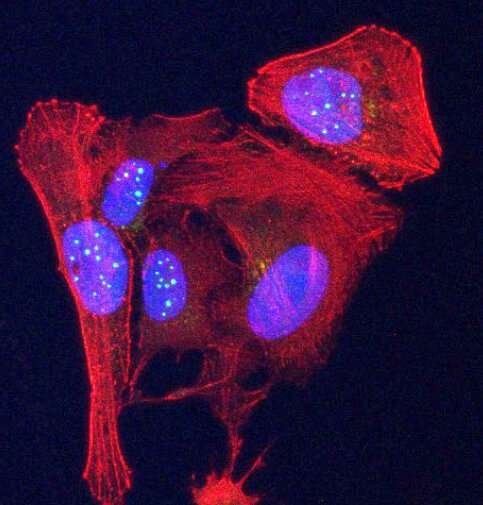
Newly discovered telomeric protein VR, (green spheres) is seen accumulating in nuclei (blue ovals) in human osteosarcoma cancer cells stained in red. (Griffith Lab)
In an article published by the UNC School of Medicine, he said the study’s finding may dramatically advance medicine’s work in cancer and aging.
“Discovering that telomeres encode two novel signaling proteins will change our understanding of cancer, aging, and how cells communicate with other cells,” he said. “Many questions remain to be answered, but our biggest priority now is developing a simple blood test for these proteins. This could inform us of our biological age and also provide warnings of issues, such as cancer or inflammation.”
Telomeres were first identified about 80 years ago. Since then, scientists have believed they could not encode for any proteins. Conventional wisdom has focused on the fact that telomeres shorten when cells divide, and eventually get so short that the cell can no longer divide properly, leading to its death.
The UNC team conducted experiments to show that telomeric DNA can instruct the cell to produce signaling proteins they termed VR (valine-arginine) and GL (glycine-leucine). These signaling proteins are chemicals that trigger a chain reaction of other proteins inside cells that then lead to a biological function important for health or disease.
“We think it’s possible that as we age, the amount of VR and GL in our blood will steadily rise, potentially providing a new biomarker for biological age as contrasted to chronological age,” said study co-author Taghreed Al-Turki, a researcher in the Griffith lab. “We think inflammation may also trigger the production of these proteins.”
The research promises to add another layer of nuance to our understanding of the aging process.