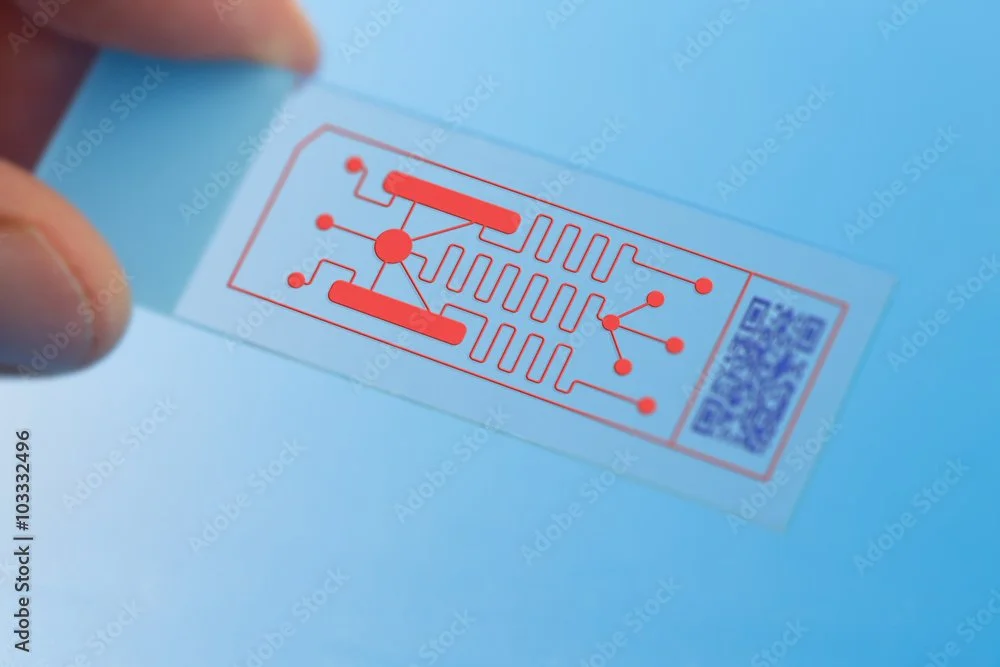
Progress on a better alternative to animal testing
/Medicine depends on animal testing to assess drug safety and efficacy. The problem is that animal tests often fail to predict clinical responses. This means drugs that look promising in animal studies often fail to deliver in human clinical trials. In fact, one study found that more than 86% of drugs found to be safe and effective in animal studies fail to get FDA approval. A new article in Nature Biomedical Engineering reports on the creation of what may eventually lead to a replacement for animal testing.
Read More